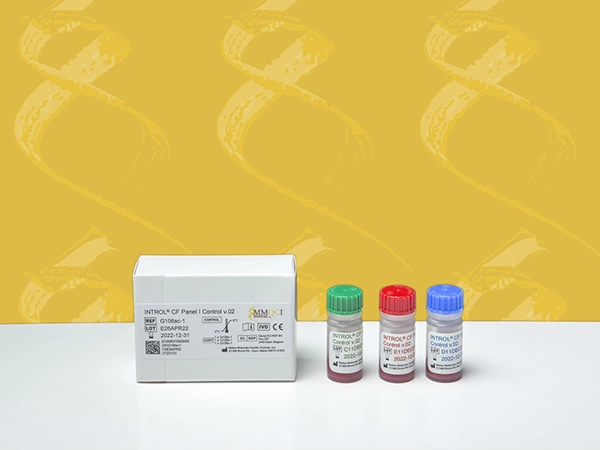
INTROL® CF Panel I Control v.02
INTROL® CF Panel I Control v.02 is intended for in vitro diagnostic use as a quality control to monitor analytical performance of the extraction, amplification and detection steps of diagnostic assays used in the detection of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) gene mutations and variants. This product is intended to be extracted and analyzed routinely with each CFTR assay run.
The INTROL CF Panel I Control v.02 is designed to monitor the detection of 38 CFTR mutations associated with cystic fibrosis, including the 23 mutations recommended for testing by American College of Medical Genetics (ACMG) and American College of Obstetricians and Gynecologists (ACOG). The INTROL CF Panel I Control also monitors 3 polymorphisms (I506V, I507V, F508C) and the 5/7/9T variants.
Product Information:
INTROL® CF Panel I Control v.02
Part Number: G106ac
GTIN: 00852720008015
Kit Contains: 3 bottles x 2mL
Controls/Qty: 1 x G106a, 1 x G106b, and 1 x G106c
Storage: Refrigerated (2° - 8°C)
Part Number: G106ac-1
GTIN: 00852720008008
Kit Contains: 3 bottles x 1mL
Controls/Qty: 1 x G106a-1, 1 x G106b-1, and 1 x G106c-1
Storage: Refrigerated (2° - 8°C)
Documents:
You might be interested in: